A gut-brain neuromodulation approach for patients 8 years and older with functional abdominal pain associated with IBS or functional dyspepsia (FD).
Functional abdominal pain associated with IBS and FD is believed to involve abnormal processing between the brain and the gut, otherwise known as the “brain-gut axis”.
IB-Stim targets the central nervous system by sending gentle electrical impulses through the peripheral cranial nerve branches located just beneath the skin in the ear. This results in neuromodulation of key brain and autonomic pathways involved in pain processing and gut–brain signaling.
Mechanism of IB-Stim supported by peer-reviewed literature:
- Pre-clinical electrophysiology study demonstrates modulation of the amygdala1, an important area involved in emotional pain processing, autonomic regulation, and chronic pain states 2,3.
- Clinical studies in adolescents with functional abdominal pain show improvement in vagal efficiency, reflecting enhanced parasympathetic regulation4,5.
- Neuroimaging studies in adults further demonstrate resetting of brain network connectivity, including the default mode network, which is implicated in chronic pain and central sensitization6.
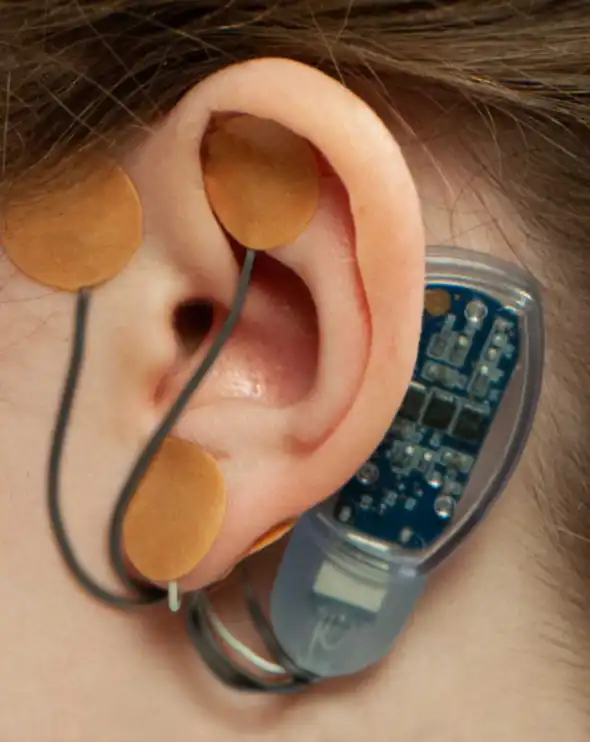
Activity of neurons in the Amygdala is decreased after 15 minutes electrical stimulation*
65% Reduction In Amygdala Firing.
*Based on pre-clinical study using the exact same parameters of stimulation (3.2V with 1 and 10 Hz) Babygirlja R, et.al., Neuroscience. 2017;356:11-21/ #p<0.05 compared to baseline.
IB-Stim and functional abdominal pain in adolescents – how does it work?
Important Safety Information
Indications For Use
The IB-Stim is a percutaneous electrical nerve field stimulator (PENFS) system intended to be used in patients 8 years and older with functional abdominal pain associated with irritable bowel syndrome (IBS) and functional dyspepsia (FD). The IB-Stim is intended to be used for 120 hours per week, using 1 device per week for 4 consecutive weeks, through application to branches of Cranial Nerves V, VII, IX and X, and the occipital nerves identified by transillumination, as an aid in the reduction of pain when combined with other therapies for IBS and FD.
FD patients for whom IB-Stim is indicated have also been shown to experience reduction of nausea symptoms.
.
DeNovo Number: DEN180057/K252024
References:
- 1
- 2
- 3
- 4
- 5
- 6
Disclaimer
This information is intended for healthcare providers who may be interested in furthering research and development with PENFS, as well as payors, government bodies, and review committees seeking information about the product to help them, on a case-by-case basis, make coverage decisions.
Please note, that the research being described may include information about technology and intended uses of that technology which have not been reviewed or approved/cleared by the U.S. FDA, and is being provided for informational purposes only.
NeurAxis does not recommend or suggest the use of its PENFS IB-Stim device for uses beyond those that are cleared by the U.S. FDA.
IB-Stim is an FDA–indicated Medical Device – DEN180057/K252024. Click here for Important Information, Indications For Use, Contraindications, Warnings, Precautions, and Risks (Reported in Patients 8 Years and Older) along with Testimonial Information.
A New Standard Of Care
*The IB-Stim is a percutaneous electrical nerve field stimulator (PENFS) system intended to be used in patients 8 years and older with functional abdominal pain associated with irritable bowel syndrome (IBS) and functional dyspepsia (FD). The IB-Stim is intended to be used for 120 hours per week, using 1 device per week for 4 consecutive weeks, through application to branches of Cranial Nerves V, VII, IX and X, and the occipital nerves identified by transillumination, as an aid in the reduction of pain when combined with other therapies for IBS and FD. FD patients for whom IB-Stim is indicated have also been shown to experience reduction of nausea symptoms. DEN180057/K252024.