FDA evaluation on IB-Stim efficacy
The overall study was a double-blind, randomized, sham-controlled study that included a cohort of patients (n=104), ages 11-18 years with functional abdominal pain disorders (FAPDs) based on Rome III criteria and included subjects with irritable bowel syndrome (IBS), functional abdominal pain, functional dyspepsia and abdominal migraine.
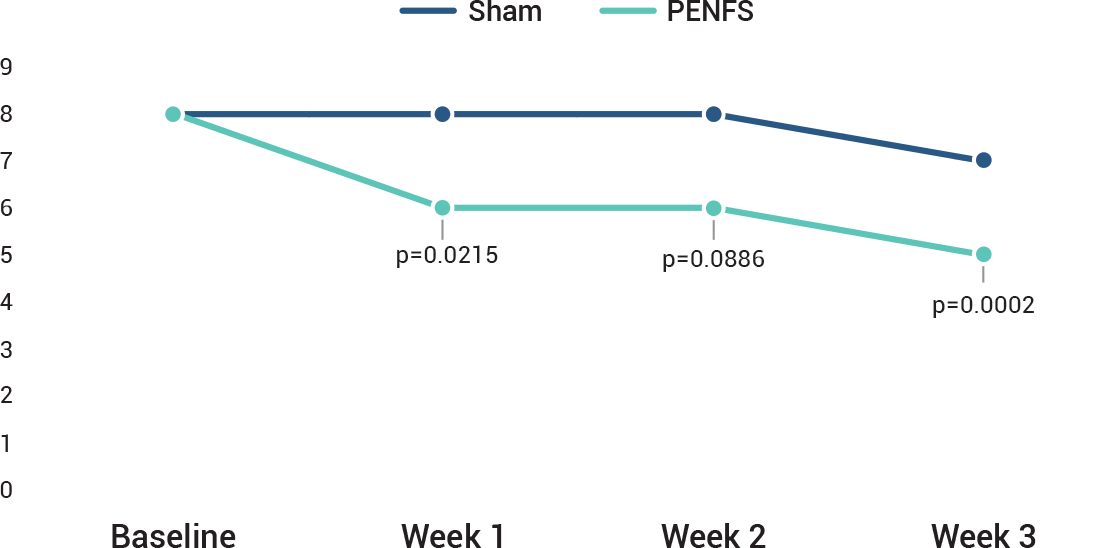
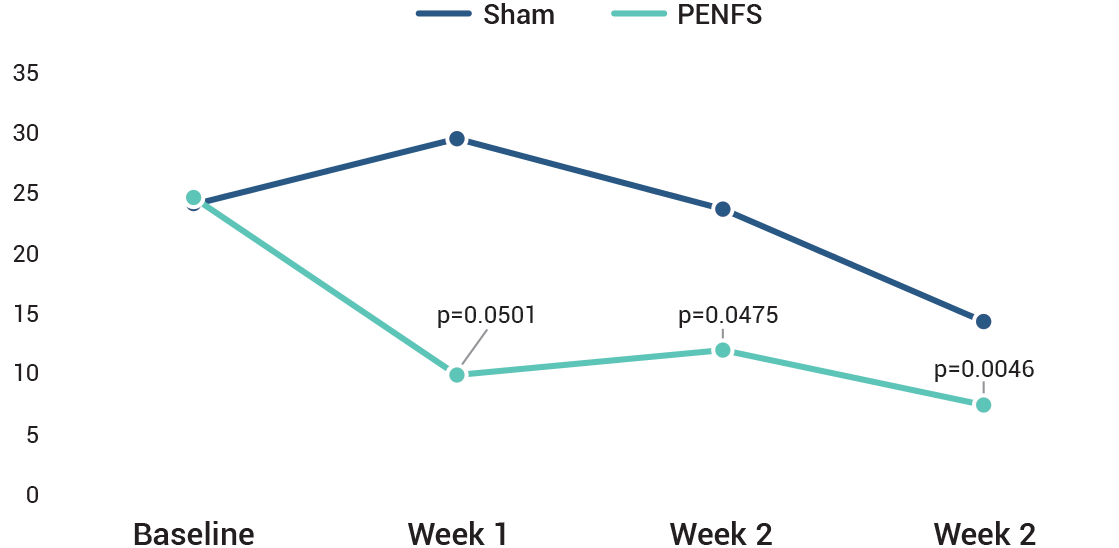
The study was designed to measure the effects of IB-Stim compared to sham on several aspects of pain including worst pain and the pain-frequency-severity-duration (PFSD) composite score. Other outcomes measured were global symptom improvement based on the Symptom Response Scale (SRS). A post-hoc analysis of ≥ 30% improvement in worst abdominal pain was also done.
In all subjects, application of IB-Stim or sham device was weekly for a total of 4 weeks and efficacy data were collected after 1, 2 and 3 full weeks. No efficacy data were collected at 4 weeks. A post-hoc sub-group analysis in a total of 50 subjects focused on the reduction of abdominal pain in subjects with irritable bowel syndrome (IBS-D, IBS-M and IBS-C).
Prior to IB-Stim treatment, 70% of patients had failed to improve with an average of 4 medications trialed
Symptom Response Scale (SRS)
Global Symptom Improvement
The Symptom Response Scale (SRS) was used to measure global symptom improvement as a secondary outcome. This scale was used to assess within-patient change over time as better, worse, or no change based on a 15-point scale across individual domains for both improvement and deterioration of overall symptoms. A global symptom rating on the symptom response scale (SRS) of +2 was considered clinically significant improvement in overall symptoms.
An improvement in the treatment group over sham was seen after 3 weeks of treatment. At the end of 3 weeks of therapy, 81% of the IB-Stim compared to 26% of the sham group reported overall symptom improvement with an SRS score of ≥2. Changes in stooling frequency during treatment with IB-Stim were not formally evaluated. For additional information on the clinical study, please see the article published by Kovacic et al. (2017).
Improvement In Abdominal Pain
IB-Stim treatment resulted in at least a 30% improvement in worst pain at the end of 3 weeks in 59% (16/27) vs 26% (6/23) in the active and sham groups, respectively. post-hoc analysis of ≥30% improvement in worst abdominal pain was also done.
In all subjects, application of IB-Stim or sham device was weekly for a total of 4 weeks and efficacy data were collected after 1, 2 and 3 full weeks.
No efficacy data were collected at 4 weeks. A post-hoc sub-group analysis in a total of 50 subjects focused on the reduction of abdominal pain in subjects with irritable bowel syndrome (IBS-D, IBS-M and IBS-C).
Symptom Response Scale (SRS)
A New Standard Of Care
*The IB-Stim is a percutaneous electrical nerve field stimulator (PENFS) system intended to be used in patients 8-21 years of age with functional abdominal pain associated with irritable bowel syndrome (IBS) and functional dyspepsia (FD). The IB-Stim is intended to be used for 120 hours per week, using 1 device per week for 4 consecutive weeks, through application to branches of Cranial Nerves V, VII, IX and X, and the occipital nerves identified by transillumination, as an aid in the reduction of pain when combined with other therapies for IBS and FD. FD patients for whom IB-Stim is indicated have also been shown to experience reduction of nausea symptoms. DEN180057.