IB-Stim Standard of Care for Functional Abdominal Pain
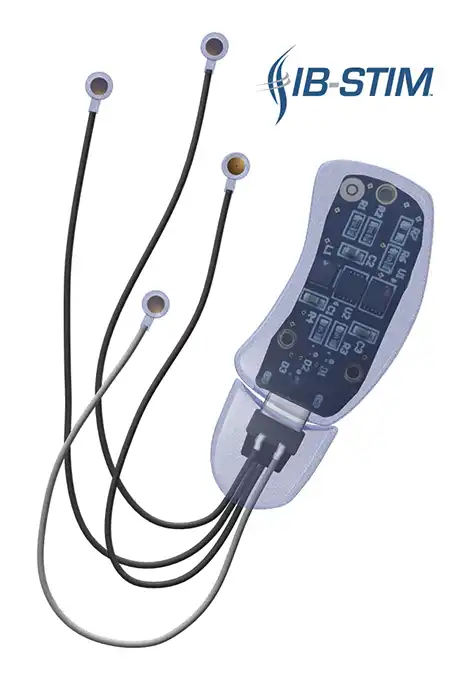
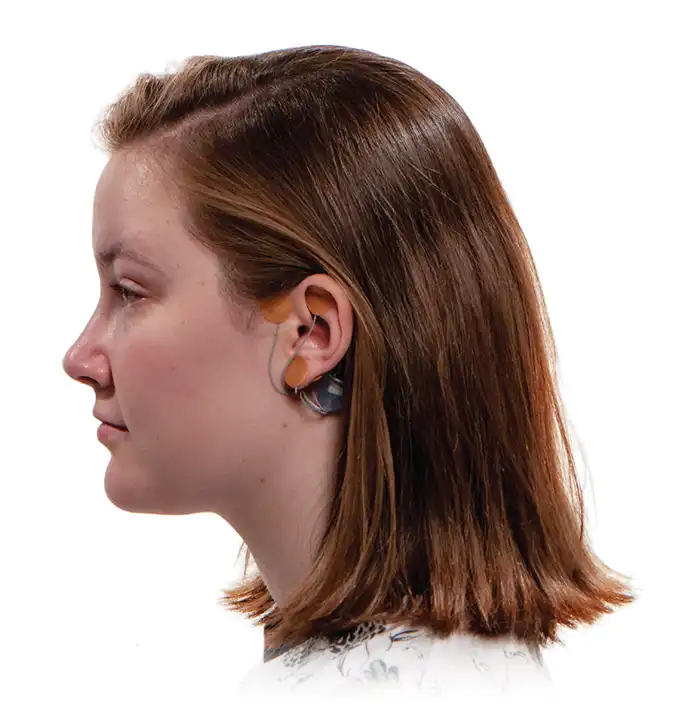
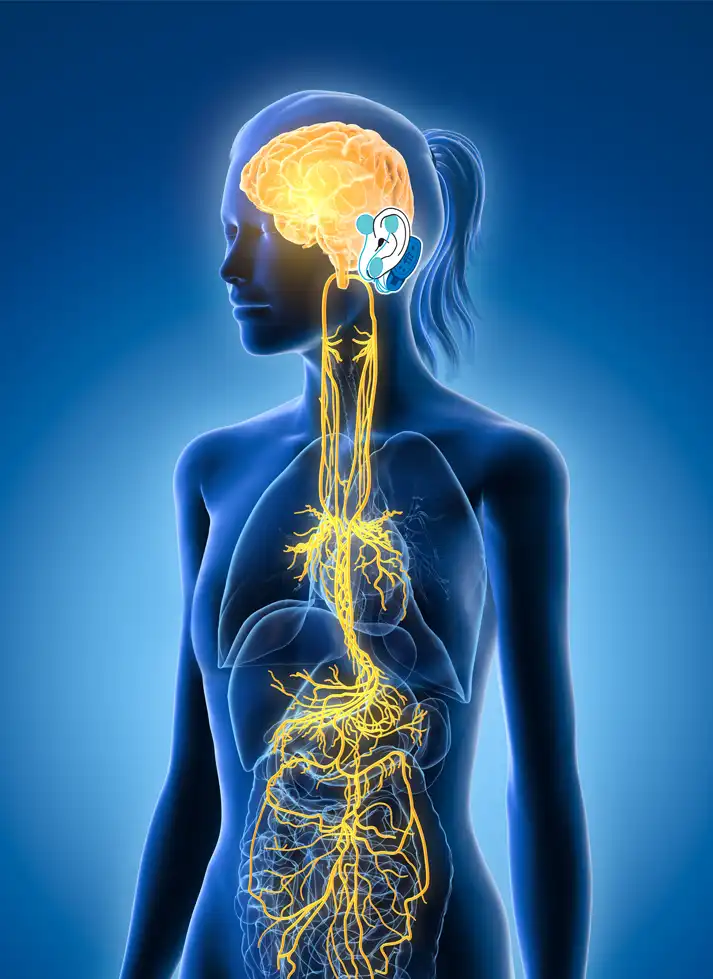
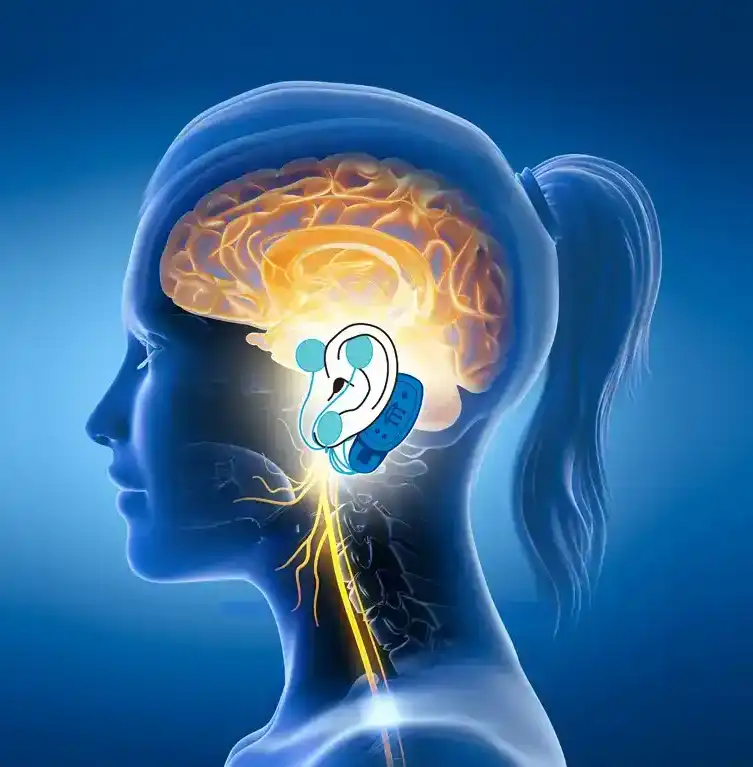
The IB-Stim is a non-surgical device that works by sending gentle electrical impulses into cranial nerve bundles located in the ear. This stimulation targets brain areas that process pain and helps reduce functional abdominal pain associated with Irritable Bowel Syndrome and functional dyspepsia. IB-Stim is a 5-day (120 hours) treatment typically worn for 4 consecutive weeks. Your healthcare provider will discuss your specific treatment plan.
At the end of the 4-week study, 95% of adolescents stated they would recommend the treatment to family or friends.
With IB-Stim, 81% of patients had improvement in global symptoms with no serious adverse events, and minimal to no side effects.
How does IB-Stim work?
Please watch our informative video for an overview of the IB-Stim process.
Will insurance cover my IB-Stim device?
The level of coverage and rules for coverage of the IB-Stim device will depend on your specific insurance policy. The IB-Stim Guidance and Patient Support (GPS) team can help you and your healthcare provider understand your insurance coverage and financial assistance options. If you have questions regarding insurance or coverage for IB-Stim, work with your healthcare provider. The IB-Stim GPS team is committed to adolescents who have been diagnosed with Functional Abdominal Pain associated with Irritable Bowel Syndrome and will assist you as needed.
A New Standard Of Care
*The IB-Stim is a percutaneous electrical nerve field stimulator (PENFS) system intended to be used in patients 8 years and older with functional abdominal pain associated with irritable bowel syndrome (IBS) and functional dyspepsia (FD). The IB-Stim is intended to be used for 120 hours per week, using 1 device per week for 4 consecutive weeks, through application to branches of Cranial Nerves V, VII, IX and X, and the occipital nerves identified by transillumination, as an aid in the reduction of pain when combined with other therapies for IBS and FD. FD patients for whom IB-Stim is indicated have also been shown to experience reduction of nausea symptoms. DEN180057/K252024.